Abstract
Shoulder resurfacing is a bone conserving method to treat advanced shoulder arthritis. By using a ceramic surface both wear and metal sensitivity are reduced to clinically unimportant levels. The inner surface of the resurfacing implant matches the native humeral head and is coated with textured surface for cementless biologic fixation. Shoulder resurfacing is of interest to young patients concerned about survivorship and those in need of a high level of physical activity. It is a versatile operation than can be used for arthritis, avascular necrosis and rotator cuff arthropathy. Since there is no stem it can be used in cases where there is deformity or prior implants in the humerus. Certain risks such as infection and instability are less common with shoulder resurfacing compared to total shoulder replacement. There is a more natural feel and patients prefer shoulder resurfacing to stemmed or stemless shoulder replacement. Shoulder resurfacing is demanding surgery that must create a close match of the surface to the native humeral head. It is a durable with only 6% of patients requiring a supplemental procedure over the next 11 years. 94% of shoulder resurfacing implants have excellent or good pain relief and functional self-reported outcomes. The ceramic surface has much less wear against articular cartilage compared to cobalt chromium and, therefore, the procedure is successful as a hemiarthroplasty. The mean wear of the native glenoid cartilage articulating with the ceramic coated shoulder resurfacing prosthesis was 0.6 mm by CT scan measurement at a mean of 10 years following implantation. In the wear simulator study this ceramic coated shoulder resurfacing implant had 30 times less wear compared to cobalt chromium. There were no patients with sensitivity to the implant. Wear debris particles were unmeasurable. Shoulder resurfacing provides excellent and mostly trouble free functional outcomes and excellent implant survivorship over the long term. 90% of patients preferred resurfacing hemiarthroplasty over conventional stemmed or stemless total shoulder replacement. Removing the humeral head is not necessary when performing an implant arthroplasty of the shoulder.
Introduction
The humeral head has been considered a surplus skeletal part and is routinely removed by most surgeons performing a shoulder implant procedure. Replacement with a metal head attached to stem placed into the shaft of the humerus has been the chosen reconstructive treatment. This may be an unnecessary concession to convention and surgeon convenience [ 11,19,20,34]. It may not be the best option for all patients. During the initial development of implant procedures surgeons were concerned about the intrusive nature of the procedures and the amount of metal involved. Biocompatibility of the implant itself was an additional concern. Any implanted material must be durable, capable of excellent functional performance and biocompatible.
Cup arthroplasty was initially suggested for both the hip and shoulder [13,14,32]. Resurfacing arthroplasty or surface replacement is an evolved technique from cup arthroplasty. In this technique replacing just the degraded surface of the humeral head and when necessary the glenoid is performed. It is easier and less demanding to excise the humeral and perform a total joint replacement but it is more conservative and may be better for the patient to perform a resurfacing procedure [1,4,6,8,16-18,25,31,36].

Resurfacing is best suited for a young patient with arthritic involvement predominately of the humeral head. It can also be used in avascular necrosis and for the occasional head splitting fracture. There are cases where resurfacing is the best option because there is deformity or prior surgery with existing hardware in the humerus blocking the placement of a stemmed prosthesis. Contraindications to resurfacing include poor bone quality or unstable soft tissues. Healthy bone is needed because the under surface of the implant is coated with a porous surface for ingrowth/ongrowth [1,4,16-18,25].
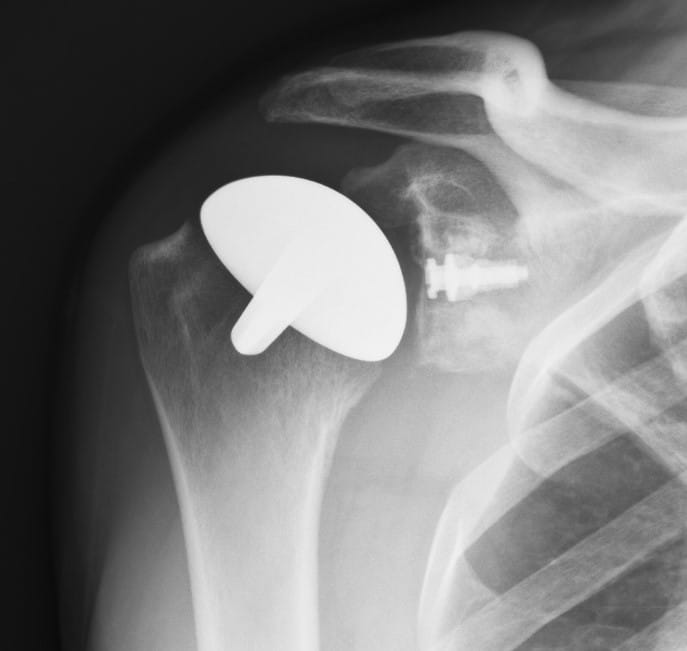
In the majority of cases the humeral resurfacing prosthesis will articulate directly with the preserved glenoid cartilage [1,4,6,16-18,25]. Therefore, the smoothest possible surface is necessary. Ceramic coating the implant is the best method to achieve this goal.[Fig. 1] Titanium Nitride (TiN) has high hardness and a low friction coefficient. It has been shown to be efficient in reducing the wear of cutting tools. It is cleared by the Food and Drug Administration as an implant bearing surface and coating. There is much less wear compared to the cobalt chromium implants that are typically used for shoulder replacement and other resurfacing implants. In addition to reduced wear, there are no metal ions released into the tissues and therefore no chance of metal sensitivity or allergic reaction [23,35,37]. There are some patients where it is possible to create enough access to place a surface on the glenoid. If needed and polyethylene is used [6,25]. [Fig. 2]
This study answered 5 questions:
- Does cementless ceramic coated shoulder resurfacing produce satisfactory function and survivorship?
- What is the wear of ceramic resurfacing prostheses?
- Can a ceramic humeral resurfacing prosthesis produce acceptable outcomes without glenoid resurfacing?
- Do patients prefer shoulder resurfacing to other implant procedures?
- Can a ceramic humeral shoulder resurfacing prosthesis provide acceptable outcomes for rotator cuff arthropathy patients?
Methods
Patient’s function and comfort were assessed with the Simple Shoulder Test (SST) before surgery and at follow-up [30,34]. Patients were followed with in person, with video visits and electronically. We used the SST because it allows the patients to assess their own function and comfort. The most recent SST score was compared to the preoperative score to assess the outcome. In addition, patients who had a stemmed or stemless prosthesis in their other shoulder were asked which shoulder they preferred. They were also asked the reasons for the preference. Most of our patients were from out of town with an average distance from the clinic of 1160 miles. The Institutional Review Board approved this study. There were 3 groups of patients:
- Humeral hemiarthroplasty
- Total resurfacing arthroplasty with a ceramic humeral resurfacing component articulating with a polyethylene glenoid
- Hemiarthroplasty for rotator cuff arthropathy
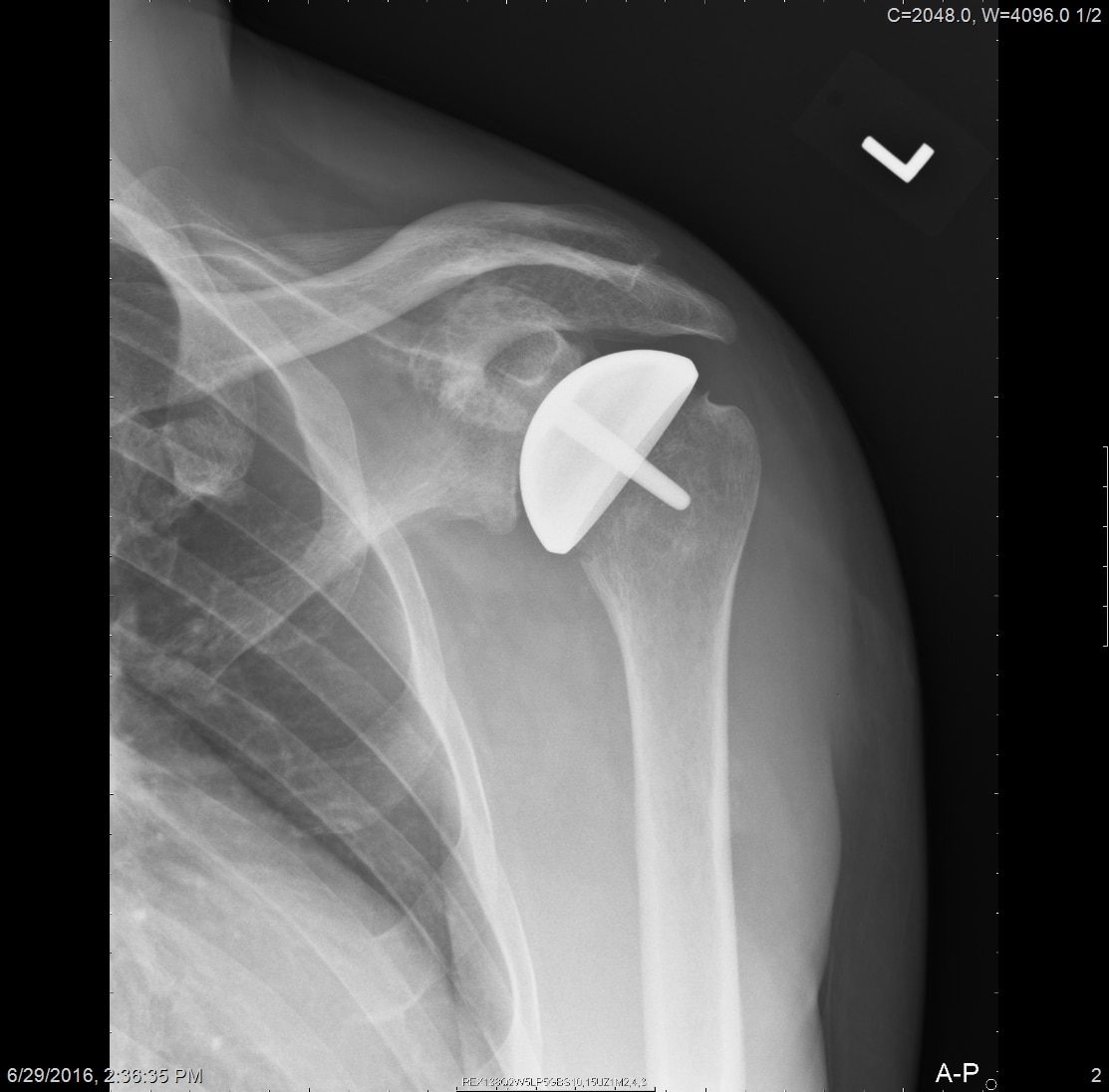
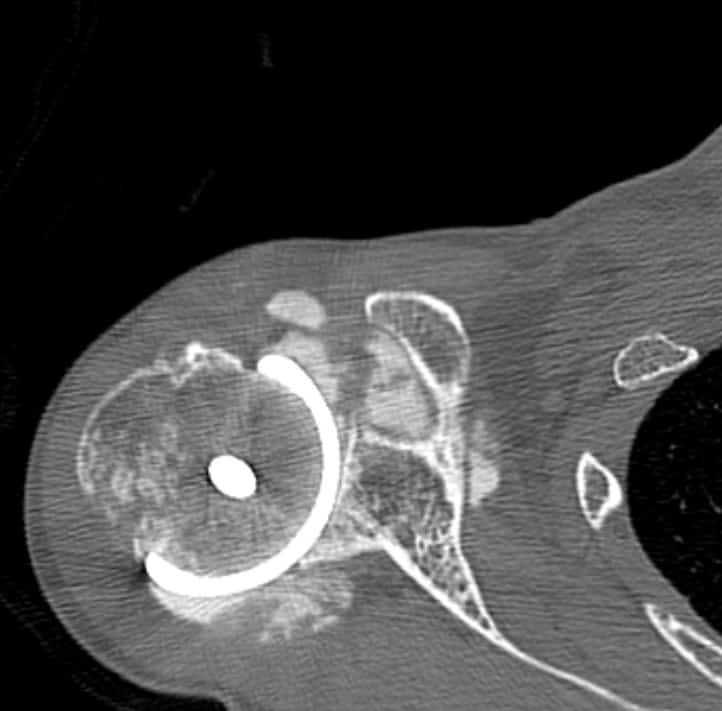
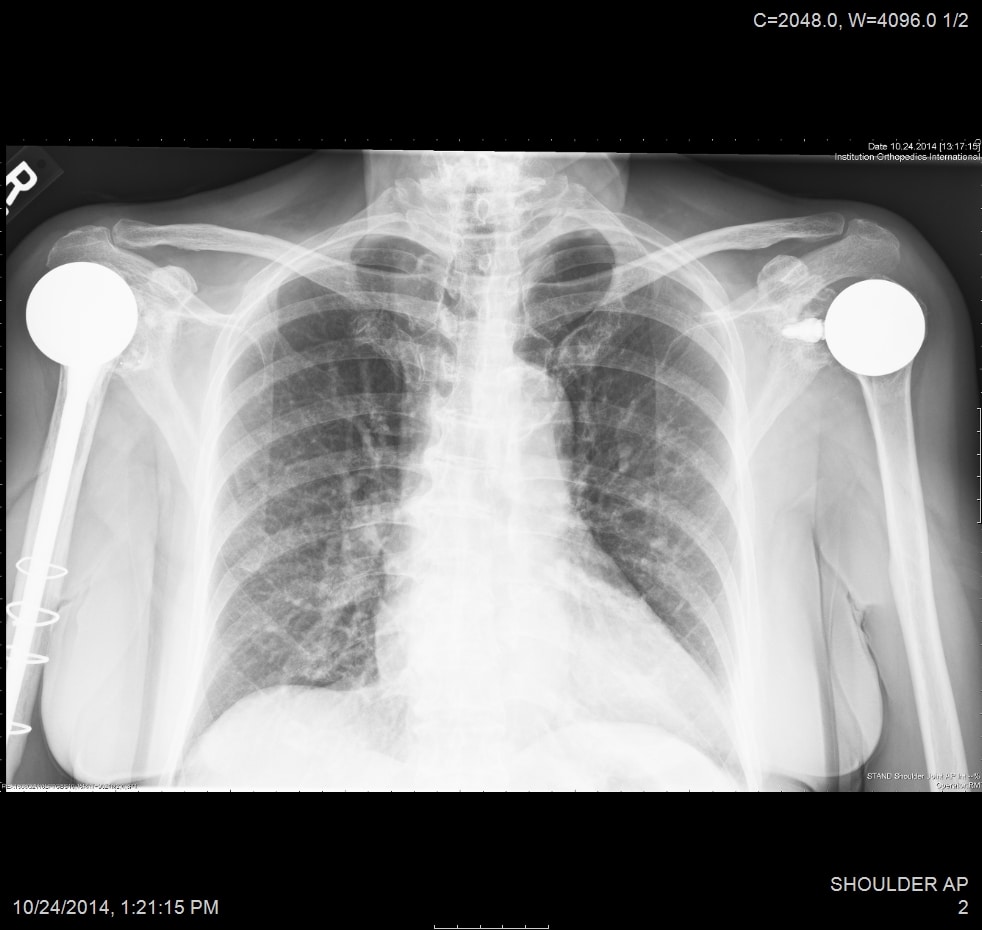
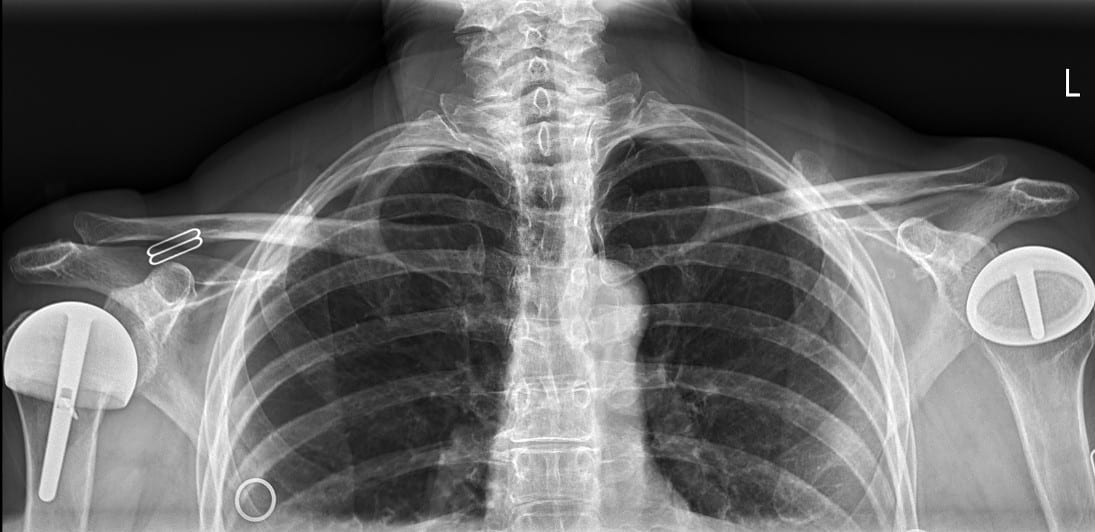
All patients received radiographs. [Fig. 3] The radiographs were examined to determine component fixation, Component position bone loss, and if evident glenoid cartilage wear. 51 hemiarthroplasty patients received CT scans 9-11 years following surgery to accurately measure the glenoid cartilage wear [7].[Fig. 4]
A wear simulator study was done. Ceramic coated resurfacing implants (8) were compared to a cobalt chromium resurfacing implants (4). The simulator was run 10 million cycles. The typical active use of a shoulder is 570,000 cycles per year so this represents 20 years of simulated use. The test was run at 1 hertz. A contact load of 765 N was chosen and the test was conducted according to ASTM F1378. The testing was conducted at shoulder joint reaction forces of 1-2 times body weight. Profilomety and microscopic examination of the humeral resurfacing component was used to identify surface damage. The implants were inspected for wear, roughness and wear particle debris generation. Retrieval analysis was performed on implants that were obtained either postmortem or if there was a revision.
The Humeral Head Implant
The titanium alloy Ti6Al4V humeral implant was straight-stemmed and proportional from 44 to 56 mm in diameter. Its undersurface was porous coated with commercially pure titanium plasma spray/beads to give an average pore size of 350 µm and a volume porosity of 30%. The articulating surface was made of titanium alloy coated with a polished 8-10 µm thick layer of titanium-nitride ceramic (TiN). This polishing was to 0.03 microns before and to 0.04 microns after the TiN coating. The TiN coating was applied by means of a Physical Vapor Deposition process [23]. When a glenoid component was used it was Ethylene Oxide sterilized GUR 1020 polyethylene.
Surgical Technique
The resurfacing arthroplasty technique was designed to restore anatomy to the articular surface of the humerus by applying a “cap” over the reamed surface of the humeral head. Ananatomic position restoring the normal degree of retroversion (average 30°) and valgus (average 140°). This allowed the tuberosity’s to be maintained with the rotator cuff attachments and a preserved force couple.
When there is rotator cuff tear arthropathy with superior migration of the humeral head but with a still captured humeral head within the coracoacromial arch resurfacing is possible. An “acetabularized” articulation with the humeral head using both the glenoid and acromion is created. With a now smooth surface can allow this articulation with altered mechanics to provide reasonable function while reducing pain. In this situation the direction of the humeral head reaming may be as high as 170 ° valgus (“hypervalgus”) and a femoral rather than humeral resurfacing device. [Fig. 5] These are limited goals cases from a functional standpoint. The arm can be raised to eye level.
For both anatomic and rotator cuff arthropathy resurfacing the operative technique is similar: a deltopectoral approach was used. A limited number of the pectoralis fibers may be released and the subscapularis is visualized. In cases of cuff tear arthropathy, the upper subscapularis may have eroded; the supraspinatus and infraspinatus are usually retracted medial to the humeral head. The humeral head rides high in the subacromial space but contained within the subacromial arch. In the more typical cases with an intact or nearly so rotator cuff the subscapularis is incised with a stump for later repair. The dissection stops at the rotator cuff interval avoiding injury to the insertion of the supraspinatus and then the head is dislocated anteriorly. In all cases great care is taken to preserve the coracoacromial ligament.
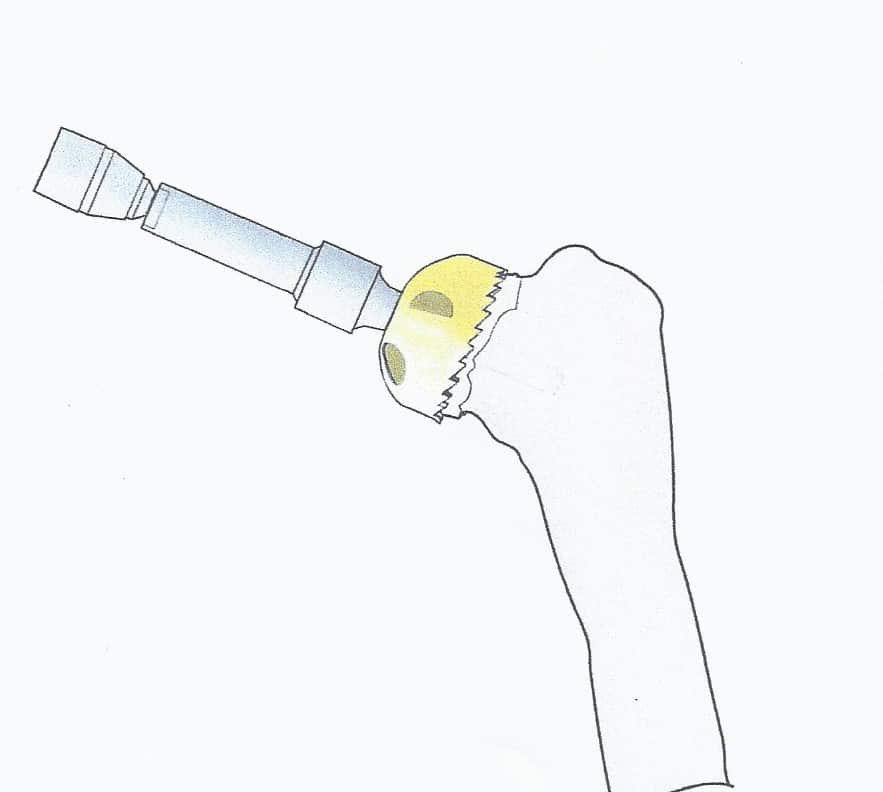
Once the humeral head has been delivered into the wound, the well-visualized peripheral osteophytes are removed. A center guide wire is placed at the normal inclination of the humeral head perpendicular to the apex of the natural articular surface. In cases of cuff tear arthropathy, the guide wire is placed in hyper valgus which will allow seating over the entire superior humeral surface including the tuberosities. If the biceps is healthy it is left intact. If not a tenodesis is performed as part of the closure. The head sizer is placed over the guide wire. Caution should be taken not to start the reamer before full application to the bone to avoid grabbing and fracturing the humeral head or neck. Only the articular surface is reamed down to bleeding bone. [Fig. 6]
Statistical Analysis
The clinical assessments were based on written responses to the Simple Shoulder Test (SST) [30,34]. These responses were collected preoperatively and postoperatively through January 2017. The percentage of maximum possible improvement was calculated for the SST score with the following formula: SST total score at the time of follow-up-SST total score preoperatively X 100%/12 points – SST total score preoperatively.
Patients were considered to have achieved a meaningful improvement if the SST increased by at least 30% of the maximum possible improvement. This method avoids the ceiling effect that results from defining as minimum clinically important differenced. Survivorship was defined as the absence of revision procedures and calculated using a Kaplan-Meier estimator.
Results
Clinical Outcomes
The outcomes were reported for three groups of patients:
- Hemiarthroplasty
- Total shoulder resurfacing with a polyethylene glenoid
- Resurfacing arthroplasty for rotator cuff arthropathy
Hemiarthroplasty
There were 428 patients. 286 (67 %) were male, 137 (32%) were female and 4 (1%) non binary. The mean patient age was 52 (standard deviation SD 10.2 years; range, 15 to 73 years). 158 (37%) had prior surgery on their shoulder. The mean preoperative SST score was 4 (SD, 2.5) out of a possible 12 shoulder functions. For the unrevised shoulder followed for a minimum of 5 years (mean 11 years; range 5 to 30 years), the mean SST score was 10.5 (SD 2.9) of 12 possible positive responses. The median SST was 11 points (interquartile range, 9 to 12 points). 402 (94%) hemiarthroplasty patients obtained ≥ 30% of the maximum possible improvement in the SST score between the preoperative and peak evaluation.26 (6%) hemiarthroplasty patients had subsequent procedures: 9 (2%) hemiarthroplasty patients had a revision to add a glenoid component, 5 revisions to total shoulder replacement, 5 revisions to a reverse total shoulder replacement, 3 subscapularis or rotator cuff repairs, 2 fracture repairs, 2 arthroscopic release procedures. There 23 incision infections with 3 deep infections. There was 1 dislocation and 2 fractures. There were 5 brachial plexopathies and one had a permanent partial median nerve deficit. There was 1 loose humeral resurfacing implant.
Total Shoulder Resurfacing
There were 91 patients treated with a total resurfacing shoulder. 51 (56%) were male, 40 (44 %) were female. The mean patient age was 64 (SD 10.3 years; range 39 to 77). 35 (39%) had prior surgery. The mean preoperative SST score was 4 (SD, 2.5). For the total shoulder arthroplasty, the mean postoperative SST score was 9.9 (SD 3.1) of 12 possible responses. The median SST was 11 points (interquartile range; 9 to 12 points). 74 patients (90 %) obtained ≥30% of the maximum possible improvement in the SST score. 9 (12%) total shoulder resurfacing patients had revision procedures. 3 were to a reverse total shoulder arthroplasty, 3 were glenoid revisions for loosening (in one instance the glenoid prosthesis was removed and not replaced. 2 were rotator cuff repairs and one was an arthroscopic lysis of adhesions. There were 2 incision infections and no deep infections. There was one brachial plexopathy that resolved.
Rotator Cuff Arthropathy
For the 67 rotator cuff arthropathy patients the age mean patient age as 67 (SD 10.6 years, range; 51 to 83 years). 46 (68%) were male and 21 (32%) were female. 44 (67%) had prior surgery. The mean preoperative SST score for rotator cuff arthropathy was 2.5 (SD, 2.5) out of a possible 12. For the rotator cuff arthropathy patients, the mean SST score was 7.7 (SD 4) of 12 possible responses. The median SST was 8 (interquartile range; 5 to 11 points). 43 (64 %) obtained ≥30% of the maximum possible improvement in the SST score. There were 3 incision infections and one deep infection in a multiply operated shoulder. There were 3 brachial plexopathies and 1 had a permanent medial nerve deficit. Of the combined group 31 patients were lost to follow-up and the outcomes at a minimum of 5 years were reported for. 19 patients died.
Patient Preference
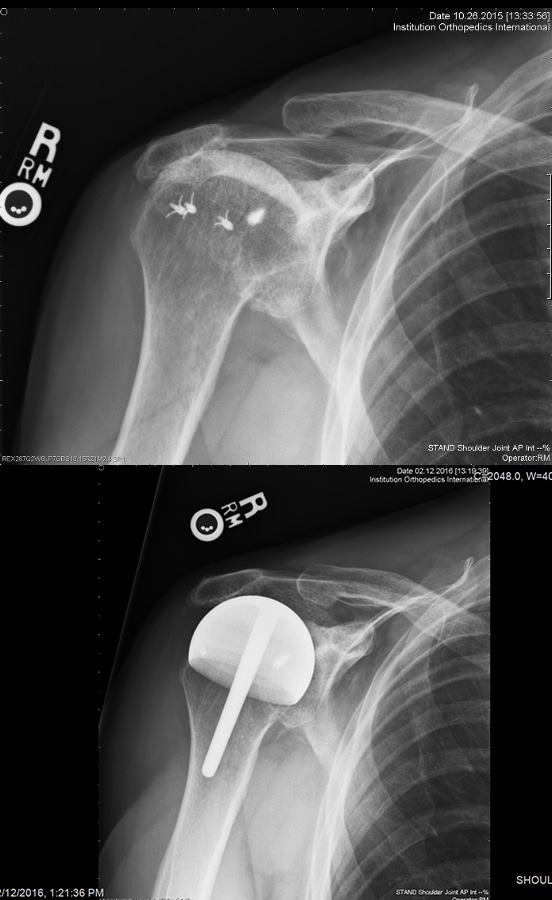
55 resurfacing hemiarthroplasty patients had a different prosthesis on the other side: a stemmed total shoulder replacement (19), stemmed hemiarthroplasty (18), total shoulder resurfacing (9) and reverse shoulder arthroplasty (7), resurfacing hemiarthroplasty for rotator cuff arthropathy (2). 11 total shoulder arthroplasty patients had a different prosthesis on each side: stemmed total shoulder replacement (5), stemmed hemiarthroplasty (3), resurfacing, hemiathroplasty (2) reverse total shoulder arthroplasty (1). [Fig. 7] 9 rotator cuff arthropathy patients had a different prosthesis on the other side: stemmed total shoulder arthroplasty (3), reverse total shoulder arthroplasty (2), stemmed hemiarthroplasty (2), resurfacing hemiarthroplasty (2). [Fig. 8]
50 of 55 (90%) of hemiarthroplasty patients preferred their hemiarthroplasty compared to their other shoulder. 3 preferred their total resurfacing shoulder or stemmed total shoulder and 2 had no preference. 8 of the 11 total shoulder resurfacing patients preferred their total shoulder resurfacing to their other shoulder. 2 preferred their resurfacing hemiarthroplasty and 1 had no preference. 6 of 9 rotator cuff arthropathy patients preferred their hemiarthroplasty to their other prosthesis and 3 preferred their reverse total shoulder arthroplasty. The reasons provided were consistent: feels more natural, feels more stable, can do more with it. [27,28] Resurfacing hemiarthroplasty took a mean of 24 minutes longer to perform compared to stemmed hemiarthroplasty.
No patient had signs or symptoms of metal sensitivity. Titanium ion concentrations were measured in 39 patients but all were unmeasurable indicating low wear and bonding of the ceramic coating.
Radiographic Examination
Radiographs showed the humeral resurfacing implant was correctly placed in all cases. There were no implants with loosening or radiolucent lines. There were no areas of osteolysis. The glenohumeral joint space as visualized on plain films was maintained. The glenohumeral joint space was measured by CT scan on 51 hemiarthroplasty patients a mean 10 years (SD 3 years, range 5-15 years) following surgery. There was a mean decrease of 0.6 mm (range, 0-1.6). There 12 cases where patients started with less than 2 mm of joint space.
Wear simulation
The wear simulation test was run using 12 specimens to 10 million cycles. Ceramic coated titanium humeral heads (8) were compared to cobalt chromium (4) in the simulator. The surface roughness of the TiN head was 85 ±55 nm and for the Cobalt chromium was 3714 ±1679 nm after 10 million cycles. The maximum wear patch in the TiN was 1.5 µm compared to pretest the thickness of the coating of 8 µm. The wear penetration, changes to surface roughness and wear particle analysis showed more than 30 times less wear with ceramic coated titanium compared to cobalt chromium.
Retrieval Studies
Post mortem or revision retrieval analysis was available for 9 TiN coated humeral implants obtained 10 or more years following implantation. The average TiN wear at the head pole of the spherical surface was 1.5 µm with the remaining 8 µm intact. There were 4 retrievals of polyethylene glenoid components articulating with TiN humeral components. The maximum polyethylene volumetric wear was 19 mm3/year. There were no areas of polyethylene wear through. There were no instances of reactive synovitis or osteolysis.
Discussion
Shoulder resurfacing is an effective procedure for increasing function and reducing pain. It is effective Both for high demand patients with an intact rotator cuff as well as limited goals patients with rotator cuff arthropathy. Complications from shoulder resurfacing are less frequent and less serious compared to stemmed hemiathroplasty and stemmed or stem less total shoulder replacement. Only one shoulder resurfacing implant was explanted for infection. The others resolved or were suppressed with antibiotics. Resurfacing implant survivorship is also better than for stemmed total shoulder replacement. Shoulder resurfacing “burns no bridges”. Revision to stemmed total shoulder replacement or reverse total shoulder replacement was uncommon but uncomplicated and successful when necessary [1,4,6,13,14,16-18,25,27,31,36]. Shoulder resurfacing is successful as a hemiarthroplasty because glenoid wear is low.
Shoulder resurfacing is a safer and less intrusive procedure compared to total shoulder replacement or hemiarthroplasy with a stemmed or stemless implant. Humeral head resurfacing is also more effective and protective than the “ream and run” procedure. In the “ream and run” procedure the glenoid is made smooth by reaming but no glenoid implant is placed [2,10,12,30,34]. A stemmed humeral implant with a prosthetic humeral head articulates with the prepared glenoid. There are 3 reasons cementless ceramic shoulder resurfacing is a better solution than “ream and run”:
- All the bone is preserved and the anatomy is more closely restored. The exact dimension of the humeral head can be recovered and the retroversion of the shoulder is restored to the anatomic position [26].
- A Humeral stem is avoided. Stems create an abnormal load transfer across the shoulder joint with stress shielding of the humerus. Also, implantation of a stem increases the difficulties involved if a prosthetic infection occurs. Stemless implants are similar to stemmed implants in that they also produce abnormal forces on the proximal humerus [15,21]. While convenient stemless implants are not equivalent to head conserving resurfacing implants.
- A ceramic surface is biocompatible and produces less tissue reaction and wear compared to cobalt chromium.
For patients with rotator cuff arthropathy shoulder resurfacing offers a less intrusive, lower risk approach than reverse total shoulder replacement. In the event a reverse total shoulder replacement fails, reconstruction can be very difficult. The results from reverse total shoulder are quite satisfactory and often better than the limited goals shoulder resurfacing but the risks are much greater than other shoulder implant procedures. [2,3,5]
Patients prefer cementless ceramic coated shoulder hemiarthroplasty over total shoulder replacement, reverse total shoulder replacement and stemmed shoulder hemiarthroplasty. The reasons are a more natural feel and better function. Preference studies are the best way to make comparisons as all other variables are controlled [28]. Preference studies have been useful in controlling bias and have become an accepted method of making comparisons.
It is very important to use a metal free implant. The wear is less and the chance of metal sensitivity is reduced. Both the retrieval and wear simulator analysis show that a lifetime of use is expected with the use of a ceramic surface. Ceramic wear is much less compared to cobalt chromium and pyrocarbon implants can fracture. Even without functional or radiographic failure, cobalt chromium wear debris produces synovitis than can be painful. TiN particles are biocompatible and result in an increase in proliferation of cells and affinity of bone to the implant. Cobalt chromium particles cause osteolysis and chondrolysis. There is lower adhesion of bacteria to TiN surfaces compared to coated or uncoated titanium, cobalt chromium and polyethylene surfaces and, therefore, a lower infection rate [21,24,29,33,35,37]. Infection is an important patient concern following any implant arthroplasty procedure. 14.3% of patients raise a concern of incisional infection. In patients where there is no concern the deep prosthetic infection rate is 0.7%. In patients where there is some concern but no definite superficial infection the deep infection rate is 3%. In patients where there is a definite superficial infection 30% go on to develop a deep prosthetic infection [9].
The results of this study should be reviewed in the light of certain limitations. First, the procedures were all performed by a surgeon experienced in the technique. Shoulder resurfacing is a demanding and exacting technique requiring a close match of the implant to the reshaped native bone. Second, only a single cementless ceramic coated implant was used. This implant is made to demanding polishing, and coating specifications. Other implants might not perform as well. Third, most of the patients lived at a distance so we relied on the patient’s assessment of their own shoulder function and comfort using the SST. Fourth, this procedure was offered to highly motivated patients who understood the rehabilitation might be long and challenging. Part of the motivation of the patients was the potential to return to a higher level of physical activity. Fifth, there may be patient and surgeon bias in favor of resurfacing due to its bone conserving nature. For the surgeon, however, resurfacing procedures are demanding and take additional time but since they are hemiarthroplasty procedures they are compensated at a lower rate compared to total shoulder replacement. For the patient, the expectations are higher so the outcomes temper the positive bias.
In conclusion cementless ceramic coated shoulder resurfacing is a valuable procedure. It has conceptual, procedural, functional, wear and preference advantages over conventional shoulder replacement options in treating advanced articular cartilage damage in the shoulder.
References
- Bailie SD, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am 2008; 90:110-117. Doi:10.2106/JBJS.F.01552.
- Banci L, Meoli A, Hintner M, Bloch H. Wear performance of inverted non-conforming bearings in anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 2019.
- Barco R, Savvido O, Sperling J, Sanchez-Sotelo J, Cofield R. Complications in reverse shoulder arthroplasty 2017 13:72-80.
- Burgess D, McGrath M, Bonutti P, Marker D, Delanois R, Mont M. Current concepts review: shoulder resurfacing. J Bone Joint Surg Am 2009;91:1128-1238. Doi: 10.2106/JBJS.H.01082.
- Chalmers P, Rahman Z, Romeo A, Nicholson G. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014 23:a737-44
- Copeland S. The continuing development of shoulder replacement: “reaching the surface.” J Bone Joint Surg Am 2006;88:900-905.
- Egund N, Jonsson E, Lidgren L, Kelly I, Petterson H. Computed tomography of humeral cup arthroplasties. Acta Radiol 1987;28:S71-73.
- Furst M, Fink B, Ruther W. The DUROM cup humeral surface replacement in patients with rheumatoid arthritis. J Bone Joint Surg Am 2007;89:1756-1762. Doi: 10.2106/JBJS.F.01290.
- Gaine W, Romamphan N, Hussein N, Hullin M, McCreth S. Wound infection in hip and knee arthroplasty. J Bone Joint Surg Br 2000 82B:561-5
- Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am 2000;82:26-34. PMid: 10653081.
- Gurd F. Surplus parts of the skeloten. Am J Surg 1947 LXXIV: 705-720.
- Hasan SS. Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA 3rd. Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg 2002;11:432-441. Doi: 10.1067/mse.2002.125806.
- Johnson E, Egund N, Kelly I, Rydholm U, Lidgren L. Cup arthroplasty of the rheumatoid shoulder. Acta Orthop Scand 1986;57:542-546.
- Jónsson E, Lidgren L, Mjöberg B, Rydholm U, Selvic G. Humeral cup fixation in rheumatoid shoulders. Acta Orthop Scand 1990;61:116-117. PMid 236095.
- Kumar S, Sperling JW, Haidukewych GH, Cofield RH. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am 2004;86:680-689. PMid 15069130.
- Levy O, Copeland SA. Cementless surface replacement arthroplasty of the shoulder. 5- to 10-year results with the Copeland Mark-2 prosthesis. J Bone Joint Surg Br 2001;83:213-221. Doi: 10/1302/0301-620X.83B2.11238.
- Levy O, Copeland SA. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elbow Surg 2004;13(3):266-271.
- Levy O, Funk L, Sforza G, Copeland SA. Copeland surface replacement arthroplasty of the shoulder in rheumatoid arthritis. J Bone Joint Surg Am 2004;86:512-518. PMid: 14996876
- Neer CS, Articular replacement for the humeral head. J Bone Joint Surg Am 1955;37:215-228.
- Neer CS, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am 1982;64:319-337.
- Orr TE, Carter DR. Stress analysis of joint arthroplasty in the proximal humerus. J Orthop Res 1985;3:360-371. Doi: 10.1002/jor.1100030313.
- Oungoulian S, Durney K, Jones B, Ahmad C, Hung C, Ateshian. Wear and damage of articular cartilage with friction against orthopedic implant materials. J Biomech 2015 48:2958-64
- Pappas MJ, Makris G, Buechel FF. Titanium nitride ceramic film against polyethylene: A 48 million cycle wear test. Clin Orthop Relat Res. 1995; 317: 64-70.
- Parsons I, Millet P, Warner JP. Glenoid wear after shoulder hemiarthroplasty: quantitative radiographic analysis. Clin Ortho Relat Res 2004 421:120-5.
- Pritchett JW Long-Term results and patient satisfaction after shoulder resurfacing. J Shoulder Elbow Surg 2010
- Pritchett JW, Clark JM. Prosthetic replacement for chronic unreduced dislocations of the shoulder. Clin Orthop Relat Res 1987;216:89-93.
- Pritchett JW. Inferior subluxation of the humeral head after trauma or surgery. J Shoulder Elbow Surg 1997;6:356-359. Doi: 10.1016/S1058-2746(97)90003-3.
- Pritchett JW. Hip replacement or hip resurfacing with a highly cross-linked polyethylene acetabular bearing:a qualitative and quantitative preference study. J Bone Joint Surg Open Access 2020
- Reiner T, Bader N, Panzam B, Kretzer J, Pais P, Zeifan F. In vivo blood metal ion levels in patients after total shoulder arthroplasty. J Shoulder Elbow Surg 2019 28:539-546
- Roy JS, Macdermid JC, Faber KJ, Drosdowech DS, Athwal GS. The Simple Short Test is responsive in assessing change following shoulder arthroplasty. J Orthop Sports Phys Therp 2010 40:413-21
- Rydholm U. Surface replacement of the humeral head in rheumatoid shoulder. J Shoulder Elbow Surg 1993;2:286-295. Doi: 10.1016/1058-2746(93)90074-Q.
- Scuderi C. Arthroplasty cup with center pin. Surg Gynecol Obst 1955 100:631-632.
- Smith S, Kennard E, Joyce T. Shoulder Simulator Test of Five Contemporary Total Shoulder Prostheses with Three Axes of Rotation and Sliding Motion Biotribology 2018.
- Somerson J, Matsen FA. Functional outcomes of the ream-and-run shoulder arthroplasty. J Bone Joint Surg AM 2017 99:1999-2003.
- Sovak G, Weiss, Gotman. Osseiointegration of Ti6Al14V alloy implants coated with titanium nitride by a new method. J Bone Joint Surg 2000 Br 82:290-296
- Steffee AD, Moore RW. Hemi-resurfacing arthroplasty of the shoulder. Contemp Orthop 1984;9:51-59.
- Van Rayy J, Rozing P, van Bitterswijk R, van Haastert M, Koerten H. Biocampatability of wear resistant coatings in orthopaedic surgery in vitro testing with human fibroblast cell cultures. J Materials Science: Materials in MedicinE 1995 6:80-84

